
TREATING ACTINIC KERATOSIS (AK) LESIONS WITH AMELUZ® (aminolevulinic acid HCl) topical gel, 10% AND BF-RhodoLED®
Choose a therapy that treats the field1,*
- AMELUZ® is indicated for the lesion-directed and field-directed treatment of actinic keratoses of mild-to-moderate severity on the face and scalp.
Treatment area should not exceed 20 cm2.
AMELUZ® (aminolevulinic acid HCl) topical gel, 10% delivers results
In a clinical study of photodynamic therapy (PDT) with AMELUZ® and BF‑RhodoLED®
(N=55)
91%
of patients were 100% cleared after 12 weeks2,3,†
Placebo
(12 weeks after the last treatment) (N=32)
22%
of patients achieved 100% clearance with placebo2,3,†

63%
of patients who were cleared after 12 weeks remained completely clear from all AK one year after the last PDT (n=50)1
- Results from a phase 3 clinical trial with 87 patients presenting 4 to 8 mild-to-moderate AK lesions on the face/forehead and/or bald scalp treated with AMELUZ® or placebo and the BF‑RhodoLED® lamp. Patients received a maximum of 2 PDTs and were examined 12 weeks and 12 months after the last treatment.1,3
Warnings:
Do not use if you have a:
- Known hypersensitivity to photoactive substances known as porphyrins.
- Known hypersensitivity to soybeans.
- Known hypersensitivity to any component of AMELUZ®.
Ask your Health Care Provider before use if you have:
- Porphyria (hereditary disease that is characterized by abnormal production of a red blood pigment called heme).
- Photodermatoses (skin conditions caused by or made worse by exposure to light or ultraviolet radiation).
When using this product:
- Allergic reactions: AMELUZ® may cause allergic reactions before photodynamic therapy. AMELUZ® should be washed off and suitable treatment started. The allergic reactions can potentially include severe courses like sudden, severe allergic reaction with breathing difficulty, swelling, lightheadedness, fast heartbeat, sweating and loss of consciousness.
- Transient Amnestic Episodes: Photodynamic therapy may cause transient amnestic episodes (temporary loss of memory). If observed, the therapy must be stopped immediately. If observed after treatment, contact your health care provider.
- Risk of Eye Injury: Patients and health care providers must wear protective eyewear while operating BF‑RhodoLED® lamp.
- Photosensitivity: Avoid sun exposure on the treated lesion sites and surrounding skin for approximately 48 hours following treatment.
- Risk of Bleeding: Special care should be taken to avoid bleeding during lesion preparation in patients with inherited or acquired coagulation disorders. Bleeding must be stopped before application of the gel.
- Ophthalmic Adverse Reactions: Avoid applying AMELUZ® into the eyes. Wash eyes with water in case of accidental contact.
- Mucous Membrane Irritation: Avoid direct contact of AMELUZ® with the mucous membranes. Wash with water in case of accidental contact.
- Concomitant use of the following medications may increase the intensity of adverse reactions after light exposure related to photodynamic therapy: St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulphonamides, quinolones, and tetracyclines.
Most common side effects at the application site were:
- skin reddening
- pain/burning
- irritation
- swelling
- itching
- scaling of the skin
- scabbing
- hardening
- blistering
Most side effects occurred during illumination or shortly afterwards, were generally of mild or moderate intensity, and lasted for 1 to 4 days in most cases; in some cases they persisted for 1 to 2 weeks or even longer.

How AMELUZ® and BF-RhodoLED® work together
AMELUZ® is used in a process called photodynamic therapy (PDT) and uses red light from a lamp called BF‑RhodoLED® to treat visible, mild-to-moderate actinic keratosis lesions on the face and scalp1 and address those hidden below the surface.2
AMELUZ®
AMELUZ® is a specially formulated gel designed to improve skin penetration and deliver the active ingredient. Within the cells this ingredient is converted into a light-activated agent. When illuminated with the red light of BF‑RhodoLED®, it creates a safe and effective therapy to destroy the premalignant cells that cause AK.1,4-6
BF‑RhodoLED®
BF‑RhodoLED® is an LED lamp that emits a deep-penetrating red light that activates the agent converted from the active ingredient of AMELUZ®.1,5,7


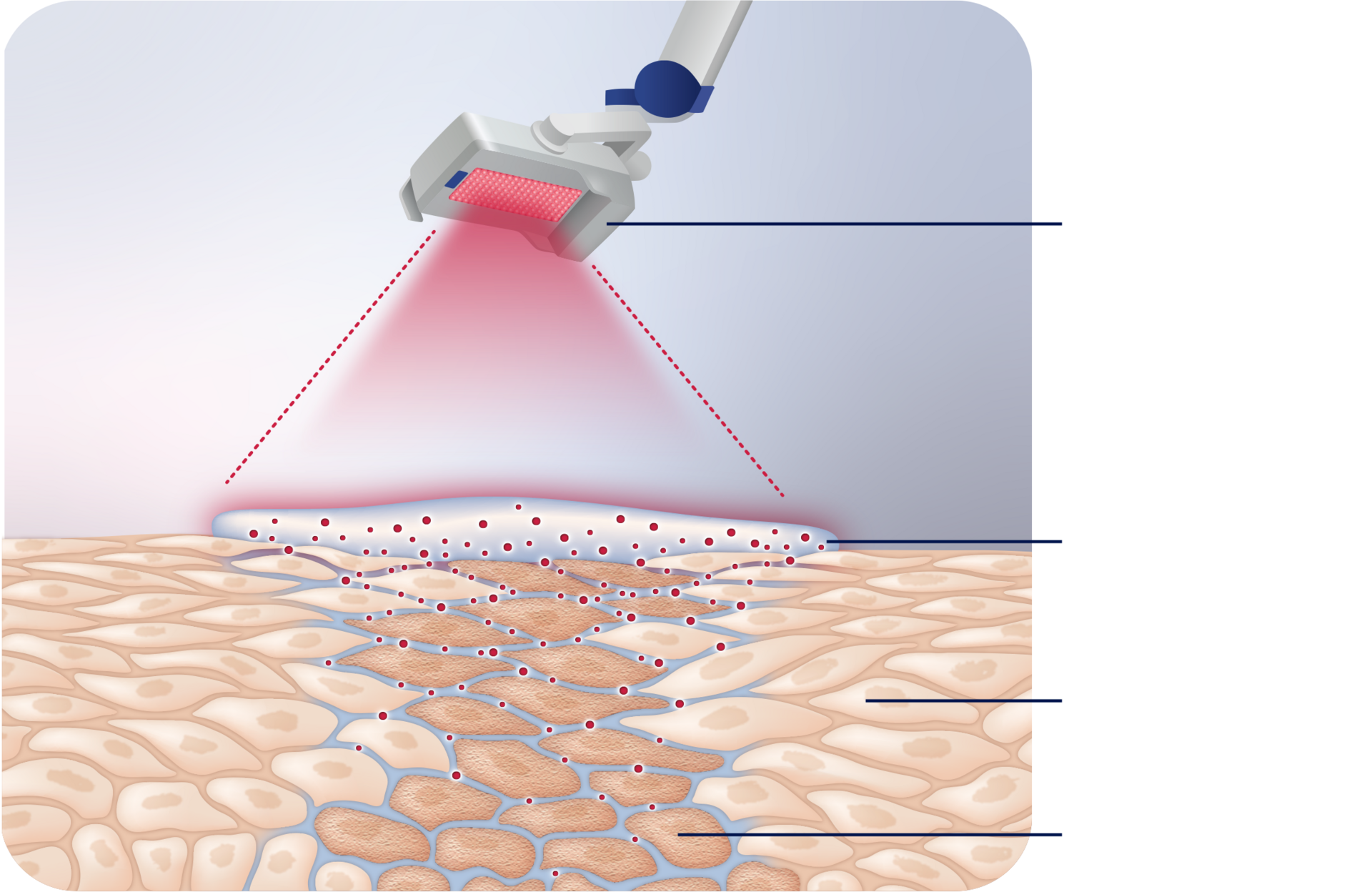
Visualization of how AMELUZ® and BF‑RhodoLED® work together
AMELUZ® penetrates the epidermis of your skin to reach the premalignant cells that cause AK. It is then converted into the light-activated agent called PpIX. The red light of BF‑RhodoLED® illuminates these cells and activates the agent, setting off a reaction that destroys the premalignant cells that cause AK—while leaving healthy skin cells mostly intact.1,2,4,5
Learn more about the treatment process with AMELUZ® and BF‑RhodoLED®.
If you have any questions about AMELUZ®, we would like to hear from you!
Call us at 1‑844‑4AMELUZ (1‑844‑426‑3589)
Or email us at info‑us@biofrontera.com

TREATING ACTINIC KERATOSIS (AK) LESIONS WITH AMELUZ® (aminolevulinic acid HCl) topical gel, 10% AND BF‑RhodoLED®
Choose a therapy that treats the field1,*
- AMELUZ® is indicated for the lesion-directed and field-directed treatment of actinic keratoses of mild-to-moderate severity on the face and scalp.
Treatment area should not exceed 20 cm2.
AMELUZ® (aminolevulinic acid HCl) topical gel, 10% delivers results
In a clinical study of photodynamic therapy (PDT) with AMELUZ® and BF‑RhodoLED®
(N=55)
91%
of patients were 100% cleared after 12 weeks2,3,†
Placebo
(12 weeks after the last treatment) (N=32)
22%
of patients achieved 100% clearance with placebo2,3,†

63%
of patients who were cleared after 12 weeks remained completely clear from all AK one year after the last PDT (n=50)1
- Results from a phase 3 clinical trial with 87 patients presenting 4 to 8 mild-to-moderate AK lesions on the face/forehead and/or bald scalp treated with AMELUZ® or placebo and the BF‑RhodoLED® lamp. Patients received a maximum of 2 PDTs and were examined 12 weeks and 12 months after the last treatment.1,3
Warnings:
Do not use if you have a:
- Known hypersensitivity to photoactive substances known as porphyrins.
- Known hypersensitivity to soybeans.
- Known hypersensitivity to any component of AMELUZ®.
Ask your Health Care Provider before use if you have:
- Porphyria (hereditary disease that is characterized by abnormal production of a red blood pigment called heme).
- Photodermatoses (skin conditions caused by or made worse by exposure to light or ultraviolet radiation).
When using this product:
- Allergic reactions: AMELUZ® may cause allergic reactions before photodynamic therapy. AMELUZ® should be washed off and suitable treatment started. The allergic reactions can potentially include severe courses like sudden, severe allergic reaction with breathing difficulty, swelling, lightheadedness, fast heartbeat, sweating and loss of consciousness.
- Transient Amnestic Episodes: Photodynamic therapy may cause transient amnestic episodes (temporary loss of memory). If observed, the therapy must be stopped immediately. If observed after treatment, contact your health care provider.
- Risk of Eye Injury: Patients and health care providers must wear protective eyewear while operating BF‑RhodoLED® lamp.
- Photosensitivity: Avoid sun exposure on the treated lesion sites and surrounding skin for approximately 48 hours following treatment.
- Risk of Bleeding: Special care should be taken to avoid bleeding during lesion preparation in patients with inherited or acquired coagulation disorders. Bleeding must be stopped before application of the gel.
- Ophthalmic Adverse Reactions: Avoid applying AMELUZ® into the eyes. Wash eyes with water in case of accidental contact.
- Mucous Membrane Irritation: Avoid direct contact of AMELUZ® with the mucous membranes. Wash with water in case of accidental contact.
- Concomitant use of the following medications may increase the intensity of adverse reactions after light exposure related to photodynamic therapy: St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulphonamides, quinolones, and tetracyclines.
Most common side effects at the application site were:
- skin reddening
- pain/burning
- irritation
- swelling
- itching
- scaling of the skin
- scabbing
- hardening
- blistering
Most side effects occurred during illumination or shortly afterwards, were generally of mild or moderate intensity, and lasted for 1 to 4 days in most cases; in some cases they persisted for 1 to 2 weeks or even longer.

How AMELUZ® and BF‑RhodoLED® work together
AMELUZ® is used in a process called photodynamic therapy (PDT) and uses red light from a lamp called BF‑RhodoLED® to treat visible, mild-to-moderate actinic keratosis lesions on the face and scalp1 and address those hidden below the surface.2
AMELUZ®
AMELUZ® is a specially formulated gel designed to improve skin penetration and deliver the active ingredient. Within the cells this ingredient is converted into a light-activated agent. When illuminated with the red light of BF‑RhodoLED®, it creates a safe and effective therapy to destroy the premalignant cells that cause AK.1,4-6

BF‑RhodoLED®
BF‑RhodoLED® is an LED lamp that emits a deep-penetrating red light that activates the agent converted from the active ingredient of AMELUZ®.1,5,7


Visualization of how AMELUZ® and BF‑RhodoLED® work together
AMELUZ® penetrates the epidermis of your skin to reach the premalignant cells that cause AK. It is then converted into the light-activated agent called PpIX. The red light of BF‑RhodoLED® illuminates these cells and activates the agent, setting off a reaction that destroys the premalignant cells that cause AK—while leaving healthy skin cells mostly intact.1,2,4,5

Learn more about the treatment process with AMELUZ® and BF‑RhodoLED®.
If you have any questions about AMELUZ®, we would like to hear from you!
Call us at 1‑844‑4AMELUZ (1‑844‑426‑3589)
Or email us at info‑us@biofrontera.com

TREATING ACTINIC KERATOSIS (AK) LESIONS WITH AMELUZ® (aminolevulinic acid HCl) topical gel, 10% AND BF‑RhodoLED®
Choose a therapy that treats the field1,*
- AMELUZ® is indicated for the lesion-directed and field-directed treatment of actinic keratoses of mild-to-moderate severity on the face and scalp.
Treatment area should not exceed 20 cm2.
AMELUZ® (aminolevulinic acid HCl) topical gel, 10% delivers results
In a clinical study of photodynamic therapy (PDT) with AMELUZ® and BF‑RhodoLED®
(N=55)
91%
of patients were 100% cleared after 12 weeks2,3,†
Placebo
(12 weeks after the last treatment) (N=32)
22%
of patients achieved 100% clearance with placebo2,3,†

63%
of patients who were cleared after 12 weeks remained completely clear from all AK one year after the last PDT (n=50)1
- Results from a phase 3 clinical trial with 87 patients presenting 4 to 8 mild-to-moderate AK lesions on the face/forehead and/or bald scalp treated with AMELUZ® or placebo and the BF‑RhodoLED® lamp. Patients received a maximum of 2 PDTs and were examined 12 weeks and 12 months after the last treatment.1,3
Warnings:
Do not use if you have a:
- Known hypersensitivity to photoactive substances known as porphyrins.
- Known hypersensitivity to soybeans.
- Known hypersensitivity to any component of AMELUZ®.
Ask your Health Care Provider before use if you have:
- Porphyria (hereditary disease that is characterized by abnormal production of a red blood pigment called heme).
- Photodermatoses (skin conditions caused by or made worse by exposure to light or ultraviolet radiation).
When using this product:
- Allergic reactions: AMELUZ® may cause allergic reactions before photodynamic therapy. AMELUZ® should be washed off and suitable treatment started. The allergic reactions can potentially include severe courses like sudden, severe allergic reaction with breathing difficulty, swelling, lightheadedness, fast heartbeat, sweating and loss of consciousness.
- Transient Amnestic Episodes: Photodynamic therapy may cause transient amnestic episodes (temporary loss of memory). If observed, the therapy must be stopped immediately. If observed after treatment, contact your health care provider.
- Risk of Eye Injury: Patients and health care providers must wear protective eyewear while operating BF‑RhodoLED® lamp.
- Photosensitivity: Avoid sun exposure on the treated lesion sites and surrounding skin for approximately 48 hours following treatment.
- Risk of Bleeding: Special care should be taken to avoid bleeding during lesion preparation in patients with inherited or acquired coagulation disorders. Bleeding must be stopped before application of the gel.
- Ophthalmic Adverse Reactions: Avoid applying AMELUZ® into the eyes. Wash eyes with water in case of accidental contact.
- Mucous Membrane Irritation: Avoid direct contact of AMELUZ® with the mucous membranes. Wash with water in case of accidental contact.
- Concomitant use of the following medications may increase the intensity of adverse reactions after light exposure related to photodynamic therapy: St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulphonamides, quinolones, and tetracyclines.
Most common side effects at the application site were:
- skin reddening
- pain/burning
- irritation
- swelling
- itching
- scaling of the skin
- scabbing
- hardening
- blistering
Most side effects occurred during illumination or shortly afterwards, were generally of mild or moderate intensity, and lasted for 1 to 4 days in most cases; in some cases they persisted for 1 to 2 weeks or even longer.

How AMELUZ® and BF‑RhodoLED® work together
AMELUZ® is used in a process called photodynamic therapy (PDT) and uses red light from a lamp called BF‑RhodoLED® to treat visible, mild-to-moderate actinic keratosis lesions on the face and scalp1 and address those hidden below the surface.2
AMELUZ®
AMELUZ® is a specially formulated gel designed to improve skin penetration and deliver the active ingredient. Within the cells this ingredient is converted into a light-activated agent. When illuminated with the red light of BF‑RhodoLED®, it creates a safe and effective therapy to destroy the premalignant cells that cause AK.1,4-6

BF‑RhodoLED®
BF‑RhodoLED® is an LED lamp that emits a deep-penetrating red light that activates the agent converted from the active ingredient of AMELUZ®.1,5,7


Visualization of how AMELUZ® and BF‑RhodoLED® work together
AMELUZ® penetrates the epidermis of your skin to reach the premalignant cells that cause AK. It is then converted into the light-activated agent called PpIX. The red light of BF‑RhodoLED® illuminates these cells and activates the agent, setting off a reaction that destroys the premalignant cells that cause AK—while leaving healthy skin cells mostly intact.1,2,4,5

Learn more about the treatment process with AMELUZ® and BF‑RhodoLED®.
If you have any questions about AMELUZ®, we would like to hear from you!
Call us at 1‑844‑4AMELUZ (1‑844‑426‑3589)
Or email us at info‑us@biofrontera.com
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
AMELUZ® (aminolevulinic acid hydrochloride) topical gel, 10%, a porphyrin precursor, in combination with photodynamic therapy using BF-RhodoLED® lamp, is indicated for the lesion-directed and field-directed treatment of actinic keratoses of mild-to-moderate severity on the face and scalp.
IMPORTANT SAFETY INFORMATION
AMELUZ® (aminolevulinic acid hydrochloride), topical gel, 10%
Purpose: Photosensitizing agent
Uses: AMELUZ® gel, a porphyrin precursor, in combination with photodynamic therapy using BF-RhodoLED® lamp, is used for lesion-directed and field-directed treatment of actinic keratoses of mild-to-moderate severity on the face and scalp.
Warnings:
Do not use if you have a:
- Known hypersensitivity to photoactive substances known as porphyrins.
- Known hypersensitivity to soybeans.
- Known hypersensitivity to any component of AMELUZ®.
Ask your Health Care Provider before use If you have:
- Porphyria (hereditary disease that is characterized by abnormal production of a red blood pigment called heme).
- Photodermatoses (skin conditions caused by or made worse by exposure to light or ultraviolet radiation).
When using this product:
- Allergic reactions: AMELUZ® may cause allergic reactions before photodynamic therapy. AMELUZ® should be washed off and suitable treatment started. The allergic reactions can potentially include severe courses like sudden, severe allergic reaction with breathing difficulty, swelling, lightheadedness, fast heartbeat. sweating and loss of consciousness.
- Transient Amnestic Episodes: Photodynamic therapy may cause transient amnestic episodes (temporary loss of memory). If observed, the therapy must be stopped immediately. If observed after treatment, contact your health care provider.
- Risk of Eye Injury: Patients and health care providers must wear protective eyewear while operating BF-RhodoLED® lamp.
- Photosensitivity: Avoid sun exposure on the treated lesion sites and surrounding skin for approximately 48 hours following treatment.
- Risk of Bleeding: Special care should be taken to avoid bleeding during lesion preparation in patients with inherited or acquired coagulation disorders. Bleeding must be stopped before application of the gel.
- Ophthalmic Adverse Reactions: Avoid applying AMELUZ® into the eyes. Wash eyes with water in case of accidental contact.
- Mucous Membrane Irritation: Avoid direct contact of AMELUZ® with the mucous membranes. Wash with water in case of accidental contact.
- Concomitant use of the following medications may increase the intensity of adverse reactions after light exposure related to photodynamic therapy: St. John's wort griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulphonamides, quinolones, and tetracyclines.
Most common side effects at the application site were:
- skin reddening
- pain/burning
- irritation
- swelling
- itching
- scaling of the skin
- scabbing
- hardening
- blistering
Most side effects occurred during illumination or shortly afterwards, were generally of mild or moderate intensity, and lasted for 1 to 4 days in most cases; in some cases they persisted for 1 to 2 weeks or even longer.
Pregnancy Warning: There is no available data on AMELUZ® use in pregnant women to inform a drug associated risk.
Lactation Warning: There is no available data regarding the presence of the active ingredient (aminolevulinic acid hydrochloride) in human milk or the effects of aminolevulinic acid hydrochloride on the breastfed infant or on milk production.
Pediatric Warning: Safety and effectiveness in pediatric patients below the age of 18 has not been established.
Geriatric Warning: No overall differences in safety or effectiveness were observed between older (65 years and older) and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Directions:
- AMELUZ® is administered only by a health care provider.
- AMELUZ® is for topical use only.
- Photodynamic therapy with AMELUZ® involves preparation of lesions, application of the product, occlusion and illumination with BF-RhodoLED®.
- Retreat lesions that have not completely resolved 3 months after the initial treatment.
Inactive Ingredients: xanthan gum, soybean phosphatidylcholine, polysorbate 80, medium-chain triglycerides, isopropyl alcohol, dibasic sodium phosphate, monobasic sodium phosphate, propylene glycol, sodium benzoate and purified water.
Other Information:
- Store in a refrigerator, 2°C – 8°C (36°F – 46°F). Excursions permitted to 15°C – 30°C (59°F – 86°F).
- The risk information provided here is not comprehensive. To learn more, talk about AMELUZ® with your health care provider. The FDA approved product labeling can be found at https://Ameluz.com/PI.
- You are encouraged to report side effects of AMELUZ®. Please contact Biofrontera Inc. at 1-844-829-7434 or FDA at 1-800-332-1088 or www.fda.gov/medwatch.
References: 1. AMELUZ [prescribing information]. Woburn, MA: Biofrontera Inc; 2021. 2. Reinhold U. A review of BF-200 ALA for the photodynamic treatment of mild-to-moderate actinic keratosis. Future Oncol. 2017;13(27):2413-2428. 3. Reinhold U, Dirschka T, Ostendorf R, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED lamp. Br J Dermatol. 2016;175(4):696-705. 4. Maisch T, Santarelli F, Schreml S, et al. Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model. Exp Dermatol. 2010;19(8):e302-305. 5. Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250-281. 6. Wu Y, Li YH, Gao XH, et al. The application of nanoemulsion in dermatology: an overview. J Drug Target. 2013;21(4):321-327. 7. Peng Q, Warloe T, Berg K, et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79(12):2282-2308.