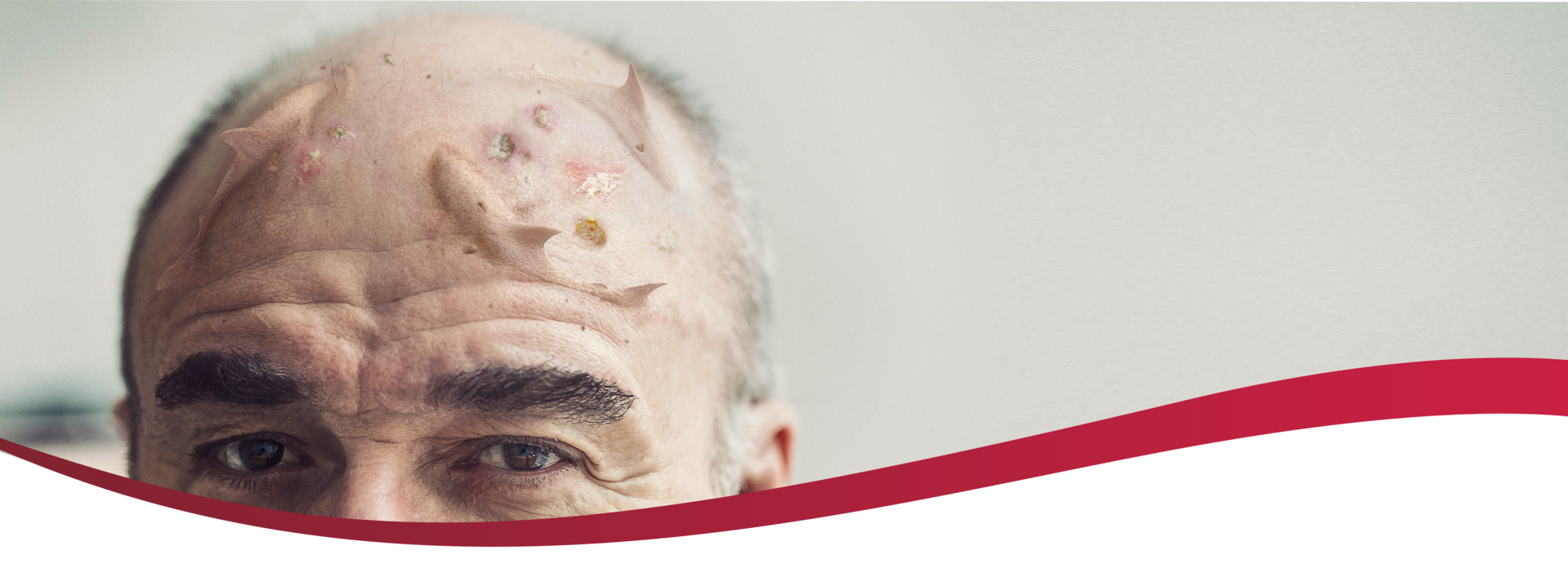
Visible actinic keratoses (AKs) often
indicate the presence of subclinical lesions.1
DANGER LURKS
BELOW THE SURFACE
AMELUZ® (aminolevulinic acid HCI) topical gel, 10% with BF‑RhodoLED® is the first and only combination product for photodynamic therapy (PDT) approved for field-directed treatment of AK of mild-to-moderate severity on the face and scalp,2 which allows subclinical lesions to also be addressed.1,3
AK lesions visible to the naked eye are markers for a sun-damaged field–an area of skin that is likely to also include premalignant AKs that are subclinical and histologically undiagnosed.1,4
Address the visible and hidden threat of AK
Discover the field-directed treatment of AK lesions of mild-to-moderate severity on the face and scalp with AMELUZ® and BF‑RhodoLED®.2,3

Schedule a PDT consult to find out how
AMELUZ® (aminolevulinic acid HCl) topical gel,
10% can benefit your patients with AK
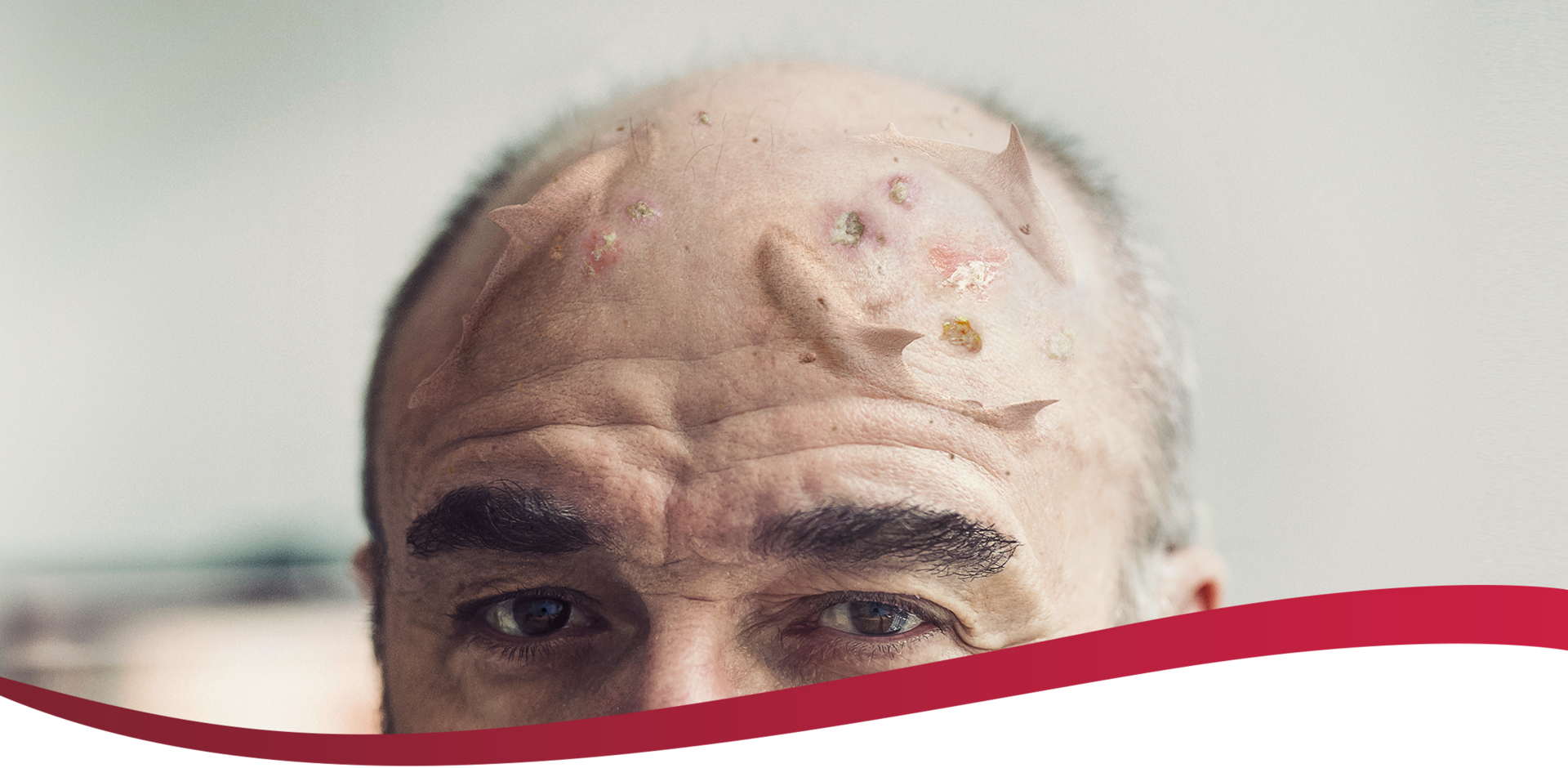
Visible actinic keratoses (AKs) often
indicate the presence of subclinical lesions.1
DANGER LURKS
BELOW THE SURFACE
AMELUZ® (aminolevulinic acid HCI) topical gel, 10% with BF‑RhodoLED® is the first and only combination product for photodynamic therapy (PDT) approved for field-directed treatment of AK of mild-to-moderate severity on the face and scalp,2 which allows subclinical lesions to also be addressed.1,3
AK lesions visible to the naked eye are markers for a sun-damaged field–an area of skin that is likely to also include premalignant AKs that are subclinical and histologically undiagnosed.1,4
Address the visible and hidden threat of AK
Discover the field-directed treatment of AK lesions of mild-to-moderate severity on the face and scalp with AMELUZ® and BF‑RhodoLED®.2,3


Schedule a PDT consult to find out how AMELUZ® (aminolevulinic acid HCl) topical gel, 10% can benefit your patients with AK
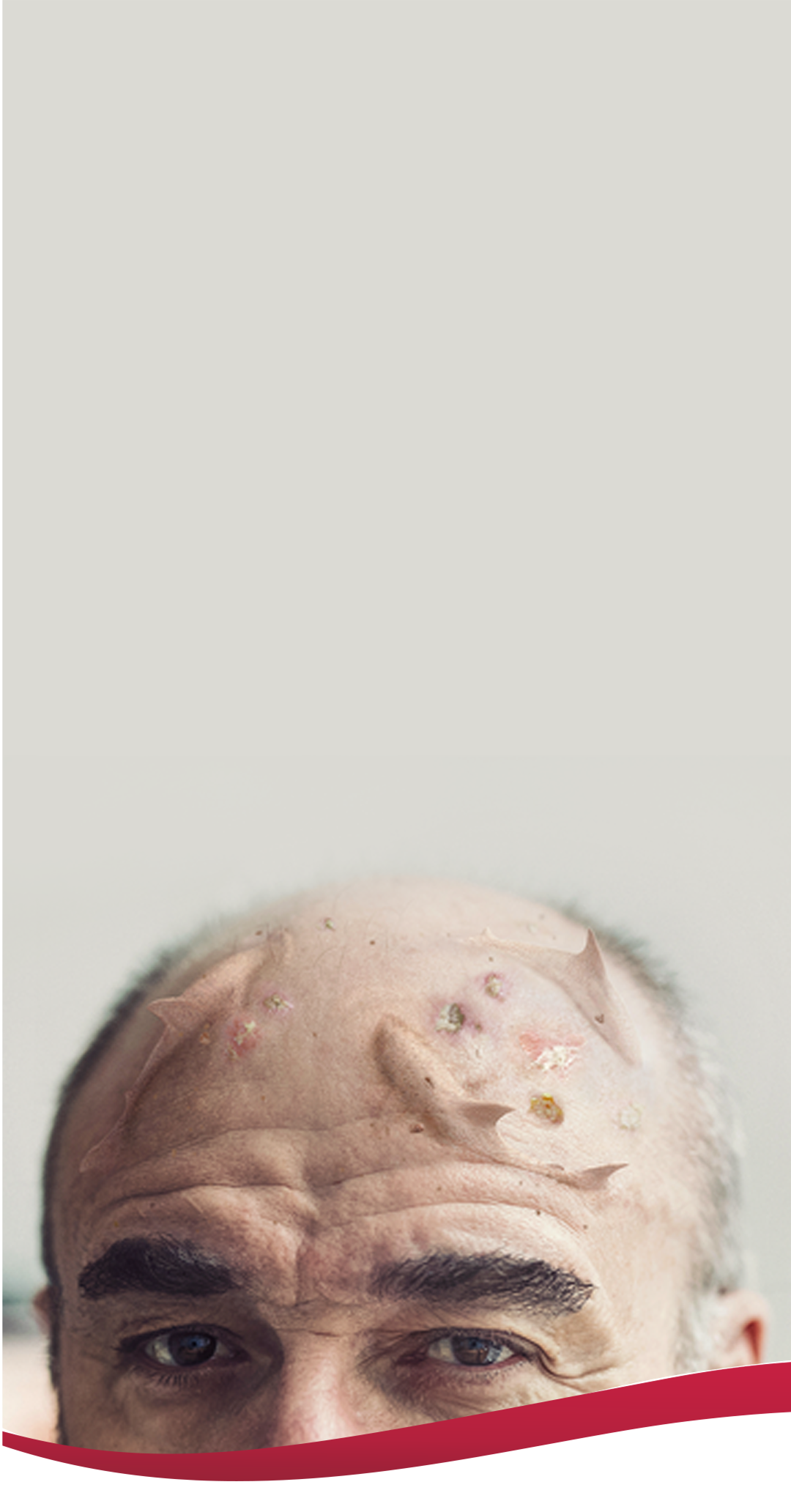
Visible actinic keratoses (AKs) often
indicate the presence of subclinical lesions.1
DANGER LURKS
BELOW THE SURFACE
AMELUZ® (aminolevulinic acid HCI) topical gel, 10% with BF‑RhodoLED® is the first and only combination product for photodynamic therapy (PDT) approved for field-directed treatment of AK of mild-to-moderate severity on the face and scalp,2 which allows subclinical lesions to also be addressed.1,3
AK lesions visible to the naked eye are markers for a sun-damaged field–an area of skin that is likely to also include premalignant AKs that are subclinical and histologically undiagnosed.1,4
Address the visible and hidden threat of AK
Discover the field-directed treatment of AK lesions of mild-to-moderate severity on the face and scalp with AMELUZ® and BF‑RhodoLED®.2,3


Schedule a PDT consult to find out how AMELUZ® (aminolevulinic acid HCl) topical gel, 10% can benefit your patients with AK
INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
AMELUZ® (aminolevulinic acid hydrochloride) topical gel, 10%, a porphyrin precursor, in combination with photodynamic therapy using BF‑RhodoLED® lamp, is indicated for the lesion-directed and field-directed treatment of actinic keratoses of mild-to-moderate severity on the face and scalp.
IMPORTANT SAFETY INFORMATION
AMELUZ® (aminolevulinic acid hydrochloride) topical gel, 10% with BF‑RhodoLED® lamp
AMELUZ®, containing 10% aminolevulinic acid hydrochloride, is a non-sterile gel formulation for topical use only. Not for ophthalmic, oral, or intravaginal use.
AMELUZ®, in conjunction with lesion preparation, is only to be administered by a health care provider. Photodynamic therapy with AMELUZ® involves preparation of lesions, application of the product, occlusion and illumination with BF‑RhodoLED®. The application area should not exceed 20 cm2 and no more than 2 grams of AMELUZ® (one tube) should be used at one time. Lesions that have not completely resolved shall be retreated 3 months after the initial treatment. Refer to BF‑RhodoLED® user manual for detailed lamp safety and operating instructions. Both patient and medical personnel conducting the PDT should adhere to all safety instructions.
AMELUZ® shall not be used by persons who have known hypersensitivity to porphyrins or any of the components of AMELUZ®, which includes soybean phosphatidylcholine. AMELUZ® should also not be used for patients who have porphyria or photodermatoses.
Hypersensitivity reactions have been reported with the use of AMELUZ® prior to photodynamic therapy (PDT). AMELUZ® should be washed off and appropriate therapy instituted. Inform patients and their caregivers that AMELUZ may cause hypersensitivity, potentially including severe courses (anaphylaxis).
Transient Amnestic Episodes have been reported during postmarketing use of AMELUZ® in combination with photodynamic therapy (PDT). If patients experience amnesia or confusion, discontinue treatment. Advise them to contact the healthcare provider if the patient develops amnesia after treatment.
Eye exposure to the red light of the BF‑RhodoLED® lamp during PDT must be prevented by protective eyewear. Direct staring into the light source must be avoided. AMELUZ® increases photosensitivity. Patients should avoid sunlight, prolonged or intense light (e.g., tanning beds, sun lamps) on lesions and surrounding skin treated with AMELUZ® for approximately 48 hours following treatment whether exposed to illumination or not.
AMELUZ® has not been tested on patients with inherited or acquired coagulation disorders. Special care should be taken to avoid bleeding during lesion preparation in such patients. Any bleeding must be stopped before application of the gel. AMELUZ® should not be used on mucous membranes or in the eyes.
Local skin reactions at the application site were observed in about 99.5% of subjects treated with AMELUZ® and narrow spectrum lamps. The very common adverse reactions (≥10%) during and after PDT were application site erythema, pain/burning, irritation, edema, pruritus, exfoliation, scab, induration, and vesicles. Most adverse reactions occurred during illumination or shortly afterwards, were generally of mild or moderate intensity, and lasted for 1 to 4 days in most cases; in some cases, however, they persisted for 1 to 2 weeks or even longer. Severe pain/burning occurred in up to 30% of treatments.
There have been no formal studies of the interaction of AMELUZ® with other drugs. Concomitant use of the following photosensitizing medications may increase the phototoxic reactions after PDT: St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulphonamides, quinolones, and tetracyclines.
There are no available data on AMELUZ® use in pregnant women to inform a drug associated risk. No data are available regarding the presence of aminolevulinic acid in human milk, the effects of aminolevulinic acid on the breastfed infant or on milk production. Safety and effectiveness in pediatric patients below the age of 18 have not been established as AK is not a condition generally seen in the pediatric population. No overall differences in safety or effectiveness were observed between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Please read the US Full Prescribing Information for AMELUZ® and/or US User Manual of BF‑RhodoLED® lamp available together at https://www.ameluz.com/PI.
You are encouraged to report side effects of AMELUZ®. Please contact Biofrontera Inc. at 1‑844‑829‑7434 or FDA at 1‑800‑332‑1088 or www.fda.gov/medwatch.
References: 1. Berman B, Amini S, Valins W, Block S. Pharmacotherapy of actinic keratosis. Expert Opin Pharmacother. 2009;10(18):3015-3031. 2. AMELUZ [Prescribing information]. Woburn, MA: Biofrontera Inc; 2021. 3. Reinhold U. A review of BF-200 ALA for the photodynamic treatment of mild-to-moderate actinic keratosis. Future Oncol. 2017;13(27):2413-2428. 4. Stockfleth E. The importance of treating the field in actinic keratosis. J Eur Acad Dermatol Venereol. 2017;31(Suppl 2):8-11. 5. Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250-281. 6. Peng Q, Warloe T, Berg K, et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79(12):2282-2308.