
PHYSICIAN SUPPORT
Access and reimbursement
Support is available to physicians offering photodynamic therapy (PDT) with AMELUZ® (aminolevulinic acid HCl) topical gel, 10% and BF‑RhodoLED®
Learn how you can integrate AMELUZ® into your practice

Reimbursement service
A FREE reimbursement service is available to help your practice check on precertification/prior authorizations to assist with any reimbursement issues.
If you have any questions regarding reimbursement for AMELUZ® or any related CPT codes, please call 1‑844‑426‑3589 or e-mail us at reimbursement@biofrontera.com and we will be happy to assist you.

Codes
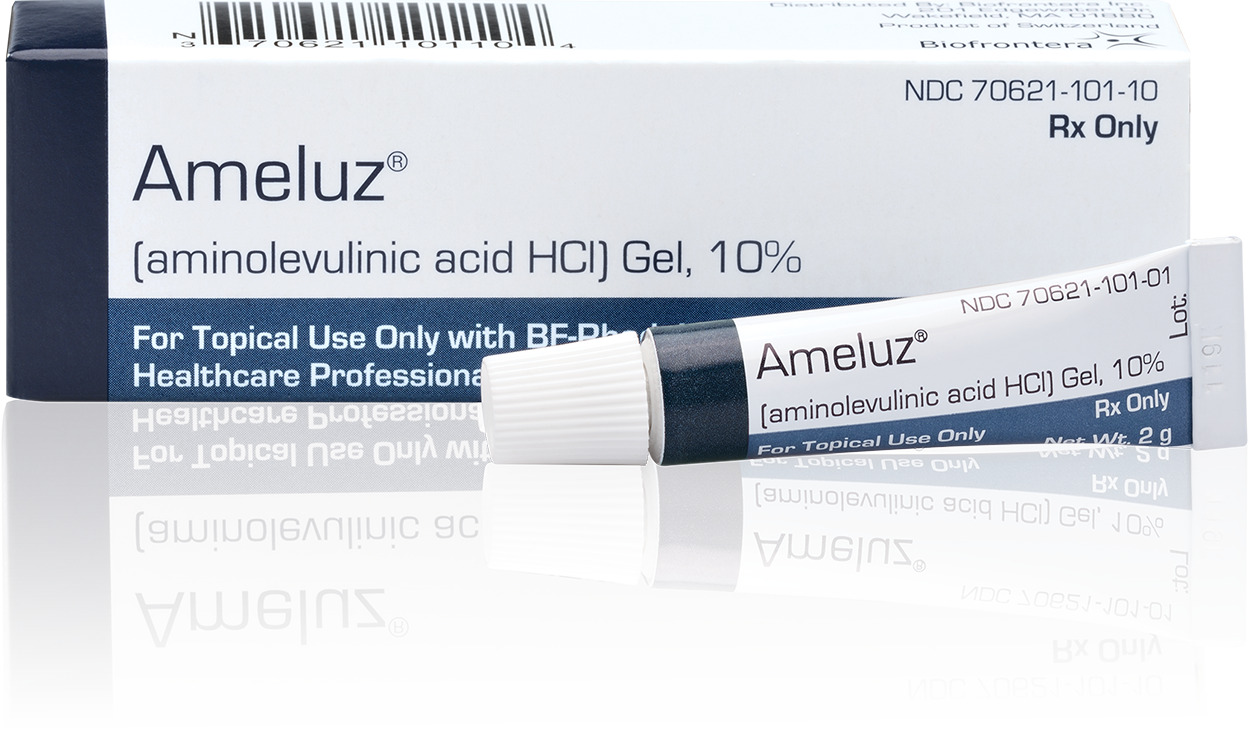
To facilitate reimbursement, codes have been assigned to both AMELUZ® and photodynamic therapy.
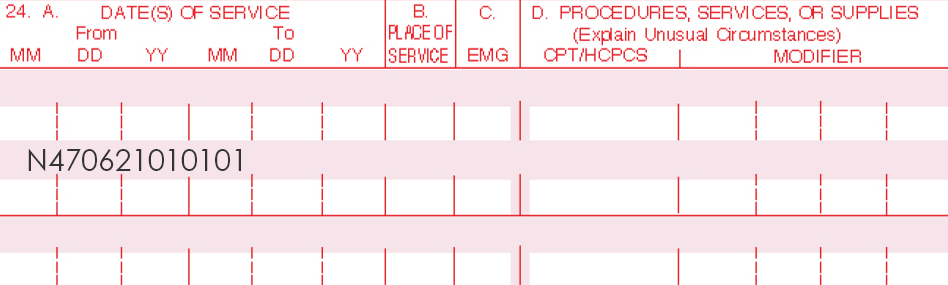
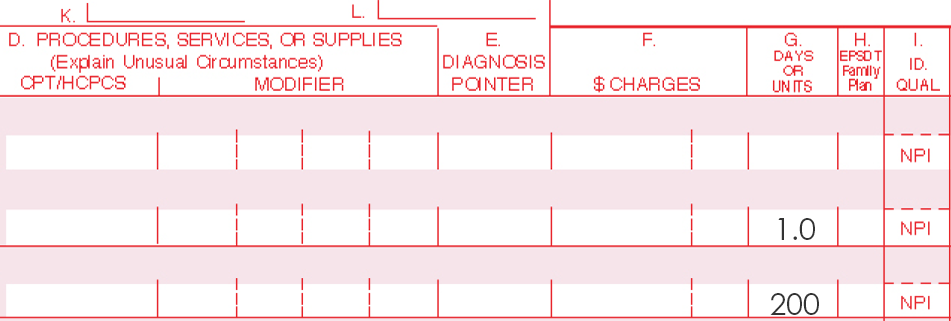
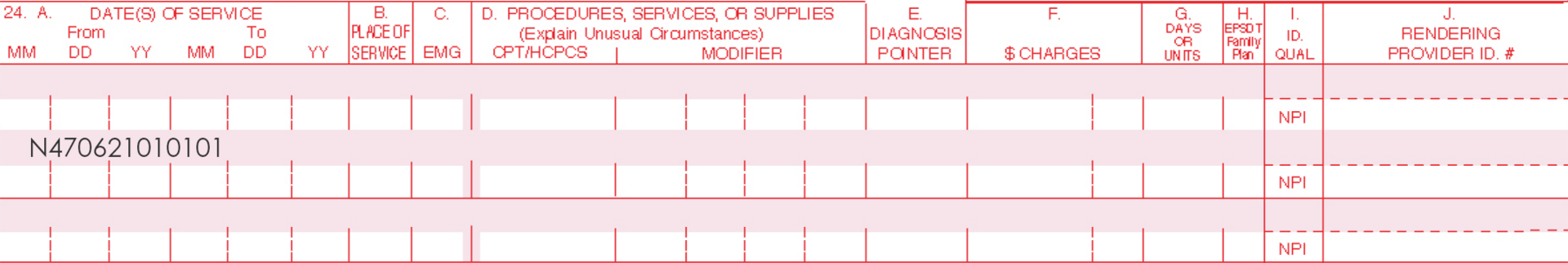
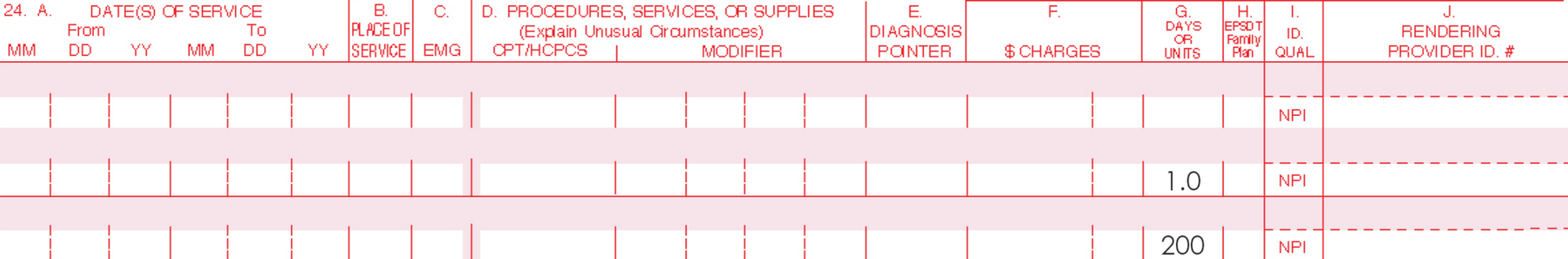
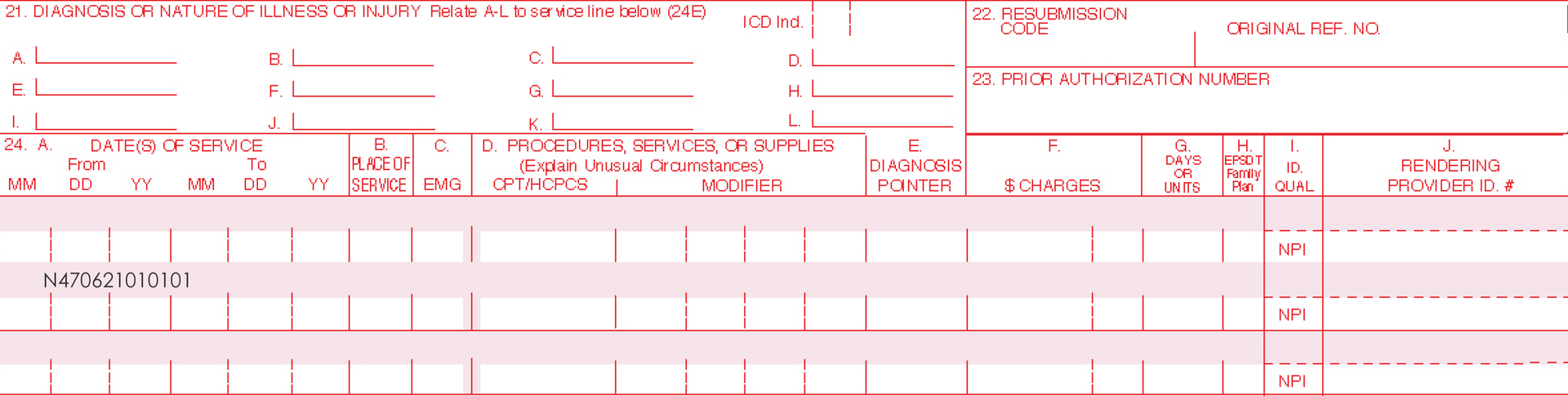
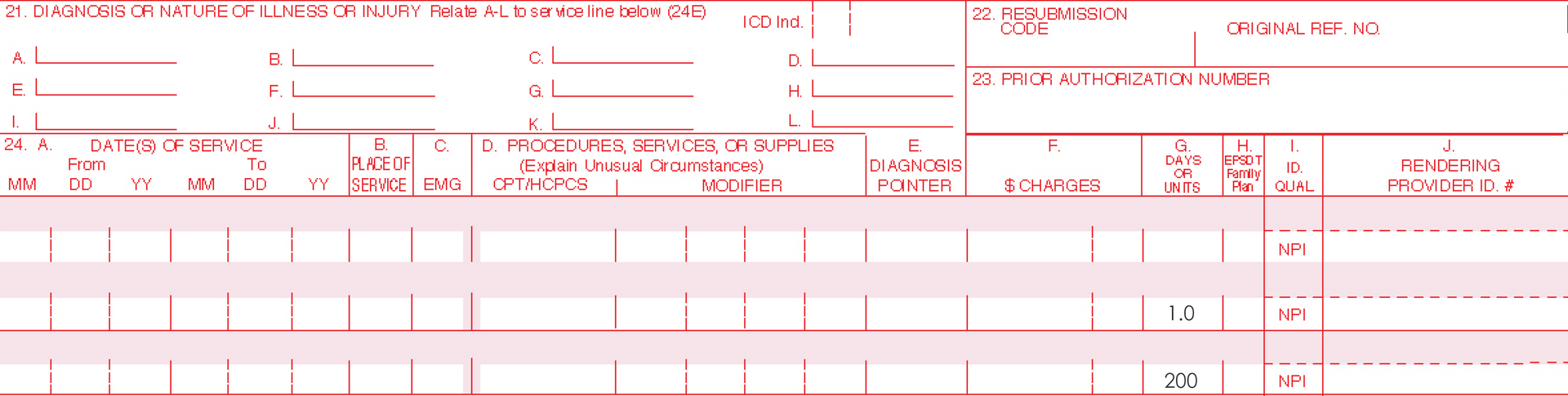
Only one Current Procedural Terminology (CPT) code can be reported per treatment, per day. For full insurance reimbursement, submit use of 200 units (one tube) of AMELUZ®.
Please be aware that the individual HCPCS code for AMELUZ® is based on 10 mg (equals 1 unit). One tube of AMELUZ® contains 2000 mg, so when using an entire tube, 200 units have to be reported in the claim form.

Current Healthcare Common
Procedure Coding System
(HCPCS) codes for
photodynamic therapy
| HCPCS Code | Descriptor |
|---|---|
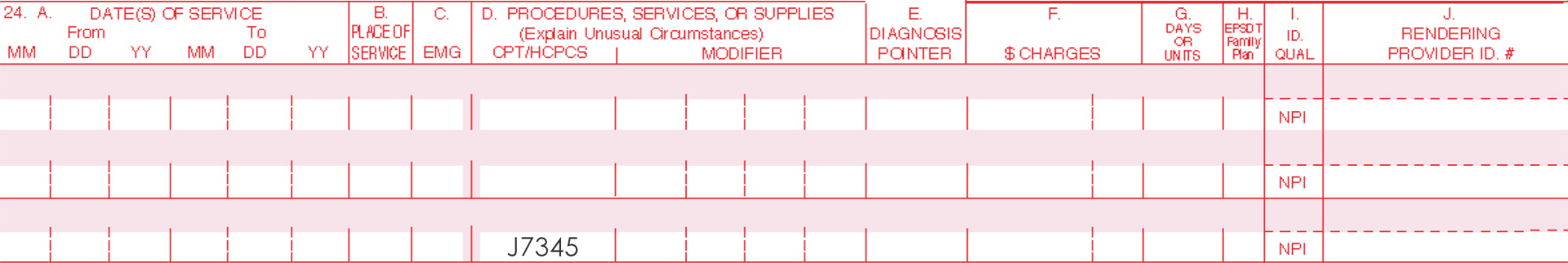
| J7345* | Aminolevulinic acid HCl gel for topical administration, 10% gel, 10 mg |
| CPT® Code |
|---|
| 96567 |
| Definition |
| Photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitive drug(s), per day |
| 2022 Medicare Physician Fee Schedule; National Average |
| $144.14 |
| CPT® Code |
|---|
| 96573 |
| Definition |
| Photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitizing drug(s) provided by a physician or other qualified health care professional, per day |
| 2022 Medicare Physician Fee Schedule; National Average |
| $234.18 |
| CPT® Code |
|---|
| 96574 |
| Definition |
| Debridement of premalignant hyperkeratotic lesion(s) (i.e., targeted curettage, abrasion) followed with photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitizing drug(s) provided by a physician or other qualified health care professional, per day |
| 2022 Medicare Physician Fee Schedule; National Average |
| $285.92 |
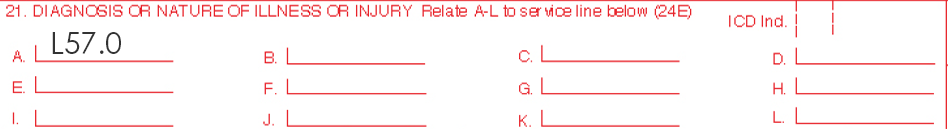
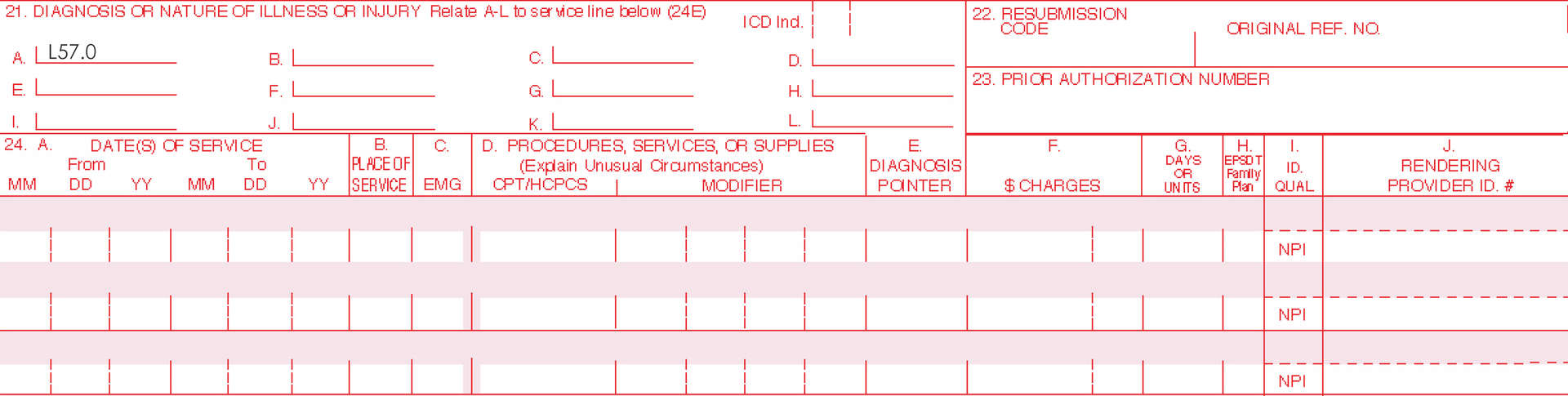
ICD-10 (International Classification of Diseases)
NDC (National Drug Code)
HCPCS (Healthcare Common Procedural Coding System)
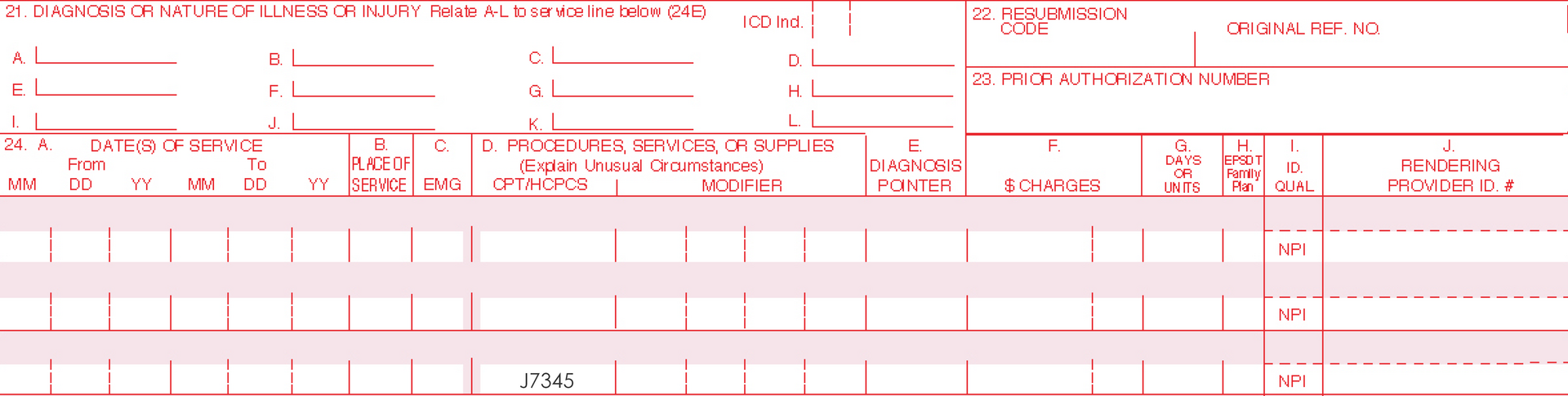
Aminolevulinic acid HCl gel for topical administration, 10% gel, 10 mg
CMS HCPCS code allowable amounts are updated on a quarterly basis. Commercial payer does not always follow the CMS reporting schedule, and rates may vary based on contractual agreements between the provider and the payer.
Units (Billable Unit Per CMS Description)
- CMS Physician Fee Schedule is available here:
https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
CPT® is a registered trademark of the American Medical Association.
Schedule a PDT consult to find out how to integrate AMELUZ® (aminolevulinic acid HCl) topical gel, 10% and BF‑RhodoLED® for the treatment of mild-to-moderate AKs on the face and scalp1 into your practice


PHYSICIAN SUPPORT
Access and reimbursement
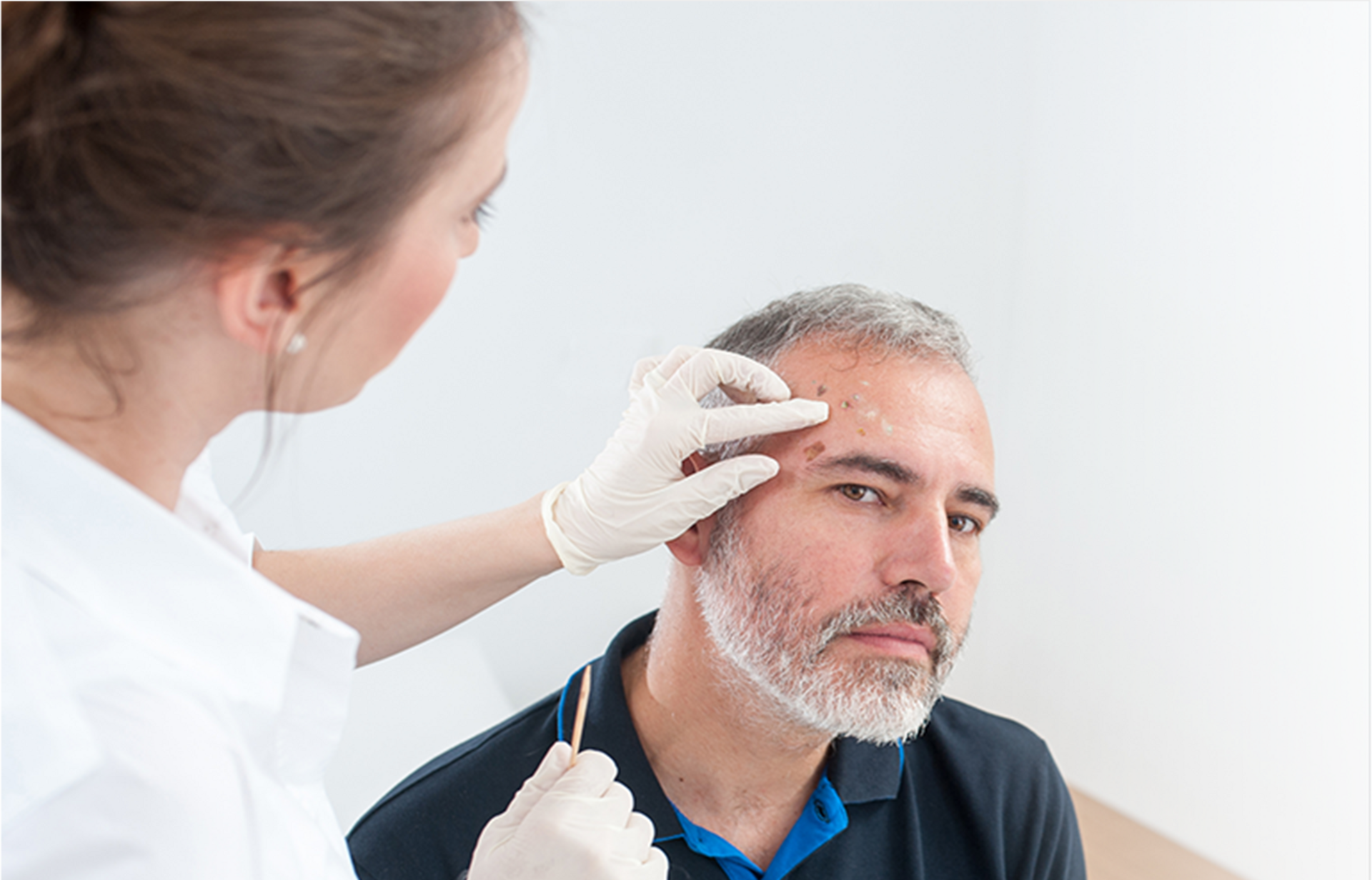
Support is available to physicians offering photodynamic therapy (PDT) with AMELUZ® (aminolevulinic acid HCl) topical gel, 10% and BF‑RhodoLED®

Learn how you can integrate AMELUZ® into your practice

Reimbursement service
A FREE reimbursement service is available to help your practice check on precertification/prior authorizations to assist with any reimbursement issues.
If you have any questions regarding reimbursement for AMELUZ® or any related CPT codes, please call 1‑844‑426‑3589 or e-mail us at reimbursement@biofrontera.com and we will be happy to assist you.

Codes
To facilitate reimbursement, codes have been assigned to both AMELUZ® and photodynamic therapy.
Only one Current Procedural Terminology (CPT) code can be reported per treatment, per day. For full insurance reimbursement, submit use of 200 units (one tube) of AMELUZ®.
Please be aware that the individual HCPCS code for AMELUZ® is based on 10 mg (equals 1 unit). One tube of AMELUZ® contains 2000 mg, so when using an entire tube, 200 units have to be reported in the claim form.

Current Healthcare Common
Procedure Coding System (HCPCS)
codes for photodynamic therapy
| HCPCS Code | Descriptor |
|---|---|
| J7345* | Aminolevulinic acid HCl gel for topical administration, 10% gel, 10 mg |
| CPT® Code | Definition | 2022 Medicare Physician Fee Schedule; National Average |
|---|---|---|
| 96567 | Photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitive drug(s), per day | $144.14 |
| 96573 | Photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitizing drug(s) provided by a physician or other qualified health care professional, per day | $234.18 |
| 96574 | Debridement of premalignant hyperkeratotic lesion(s) (i.e., targeted curettage, abrasion) followed with photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitizing drug(s) provided by a physician or other qualified health care professional, per day | $285.92 |
ICD-10 (International Classification of Diseases)
NDC (National Drug Code)
HCPCS (Healthcare Common Procedural Coding System)
Aminolevulinic acid HCl gel for topical administration, 10% gel, 10 mg
CMS HCPCS code allowable amounts are updated on a quarterly basis. Commercial payer does not always follow the CMS reporting schedule, and rates may vary based on contractual agreements between the provider and the payer.
Units (Billable Unit Per CMS Description)
- CMS Physician Fee Schedule is available here:
https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
CPT® is a registered trademark of the American Medical Association.
Schedule a PDT consult to find out how to integrate AMELUZ® (aminolevulinic acid HCl) topical gel, 10% and BF‑RhodoLED® for the treatment of mild-to-moderate AKs on the face and scalp1 into your practice


PHYSICIAN SUPPORT
Access and reimbursement
Support is available to physicians offering photodynamic therapy (PDT) with AMELUZ® (aminolevulinic acid HCl) topical gel, 10% and BF‑RhodoLED®

Learn how you can integrate AMELUZ® into your practice

Reimbursement service
A FREE reimbursement service is available to help your practice check on precertification/prior authorizations to assist with any reimbursement issues.
If you have any questions regarding reimbursement for AMELUZ® or any related CPT codes, please call 1‑844‑426‑3589 or e-mail us at reimbursement@biofrontera.com and we will be happy to assist you.

Codes
To facilitate reimbursement, codes have been assigned to both AMELUZ® and photodynamic therapy.
Only one Current Procedural Terminology (CPT) code can be reported per treatment, per day. For full insurance reimbursement, submit use of 200 units (one tube) of AMELUZ®.
Please be aware that the individual HCPCS code for AMELUZ® is based on 10 mg (equals 1 unit). One tube of AMELUZ® contains 2000 mg, so when using an entire tube, 200 units have to be reported in the claim form.

Current Healthcare Common Procedure Coding System (HCPCS)
codes for photodynamic therapy
| HCPCS Code | Descriptor |
|---|---|
| J7345* | Aminolevulinic acid HCl gel for topical administration, 10% gel, 10 mg |
| CPT® Code | Definition | 2022 Medicare Physician Fee Schedule; National Average |
|---|---|---|
| 96567 | Photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitive drug(s), per day | $144.14 |
| 96573 | Photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitizing drug(s) provided by a physician or other qualified health care professional, per day | $234.18 |
| 96574 | Debridement of premalignant hyperkeratotic lesion(s) (i.e., targeted curettage, abrasion) followed with photodynamic therapy by external application of light to destroy premalignant lesions of the skin and adjacent mucosa with application and illumination/activation of photosensitizing drug(s) provided by a physician or other qualified health care professional, per day | $285.92 |
ICD-10 (International Classification of Diseases)
NDC (National Drug Code)
HCPCS (Healthcare Common Procedural Coding System)

Aminolevulinic acid HCl gel for topical administration, 10% gel, 10 mg
CMS HCPCS code allowable amounts are updated on a quarterly basis. Commercial payer does not always follow the CMS reporting schedule, and rates may vary based on contractual agreements between the provider and the payer.
Units (Billable Unit Per CMS Description)
- CMS Physician Fee Schedule is available here: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
CPT® is a registered trademark of the American Medical Association.
Schedule a PDT consult to find out how to integrate AMELUZ® (aminolevulinic acid HCl) topical gel, 10% and BF‑RhodoLED® for the treatment of mild-to-moderate AKs on the face and scalp1 into your practice

INDICATION AND IMPORTANT SAFETY INFORMATION
INDICATION
AMELUZ® (aminolevulinic acid hydrochloride) topical gel, 10%, a porphyrin precursor, in combination with photodynamic therapy using BF‑RhodoLED® lamp, is indicated for the lesion-directed and field-directed treatment of actinic keratoses of mild-to-moderate severity on the face and scalp.
IMPORTANT SAFETY INFORMATION
AMELUZ® (aminolevulinic acid hydrochloride) topical gel, 10% with BF‑RhodoLED® lamp
AMELUZ®, containing 10% aminolevulinic acid hydrochloride, is a non-sterile gel formulation for topical use only. Not for ophthalmic, oral, or intravaginal use.
AMELUZ®, in conjunction with lesion preparation, is only to be administered by a health care provider. Photodynamic therapy with AMELUZ® involves preparation of lesions, application of the product, occlusion and illumination with BF‑RhodoLED®. The application area should not exceed 20 cm2 and no more than 2 grams of AMELUZ® (one tube) should be used at one time. Lesions that have not completely resolved shall be retreated 3 months after the initial treatment. Refer to BF‑RhodoLED® user manual for detailed lamp safety and operating instructions. Both patient and medical personnel conducting the PDT should adhere to all safety instructions.
AMELUZ® shall not be used by persons who have known hypersensitivity to porphyrins or any of the components of AMELUZ®, which includes soybean phosphatidylcholine. AMELUZ® should also not be used for patients who have porphyria or photodermatoses.
Hypersensitivity reactions have been reported with the use of AMELUZ® prior to photodynamic therapy (PDT). AMELUZ® should be washed off and appropriate therapy instituted. Inform patients and their caregivers that AMELUZ may cause hypersensitivity, potentially including severe courses (anaphylaxis).
Transient Amnestic Episodes have been reported during postmarketing use of AMELUZ® in combination with photodynamic therapy (PDT). If patients experience amnesia or confusion, discontinue treatment. Advise them to contact the healthcare provider if the patient develops amnesia after treatment.
Eye exposure to the red light of the BF‑RhodoLED® lamp during PDT must be prevented by protective eyewear. Direct staring into the light source must be avoided. AMELUZ® increases photosensitivity. Patients should avoid sunlight, prolonged or intense light (e.g., tanning beds, sun lamps) on lesions and surrounding skin treated with AMELUZ® for approximately 48 hours following treatment whether exposed to illumination or not.
AMELUZ® has not been tested on patients with inherited or acquired coagulation disorders. Special care should be taken to avoid bleeding during lesion preparation in such patients. Any bleeding must be stopped before application of the gel. AMELUZ® should not be used on mucous membranes or in the eyes.
Local skin reactions at the application site were observed in about 99.5% of subjects treated with AMELUZ® and narrow spectrum lamps. The very common adverse reactions (≥10%) during and after PDT were application site erythema, pain/burning, irritation, edema, pruritus, exfoliation, scab, induration, and vesicles. Most adverse reactions occurred during illumination or shortly afterwards, were generally of mild or moderate intensity, and lasted for 1 to 4 days in most cases; in some cases, however, they persisted for 1 to 2 weeks or even longer. Severe pain/burning occurred in up to 30% of treatments.
There have been no formal studies of the interaction of AMELUZ® with other drugs. Concomitant use of the following photosensitizing medications may increase the phototoxic reactions after PDT: St. John’s wort, griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulphonamides, quinolones, and tetracyclines.
There are no available data on AMELUZ® use in pregnant women to inform a drug associated risk. No data are available regarding the presence of aminolevulinic acid in human milk, the effects of aminolevulinic acid on the breastfed infant or on milk production. Safety and effectiveness in pediatric patients below the age of 18 have not been established as AK is not a condition generally seen in the pediatric population. No overall differences in safety or effectiveness were observed between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Please read the US Full Prescribing Information for AMELUZ® and/or US User Manual of BF‑RhodoLED® lamp available together at https://www.ameluz.com/PI.
You are encouraged to report side effects of AMELUZ®. Please contact Biofrontera Inc. at 1‑844‑829‑7434 or FDA at 1‑800‑332‑1088 or www.fda.gov/medwatch.
Reference: 1. AMELUZ [prescribing information]. Woburn, MA: Biofrontera Inc; 2021.